

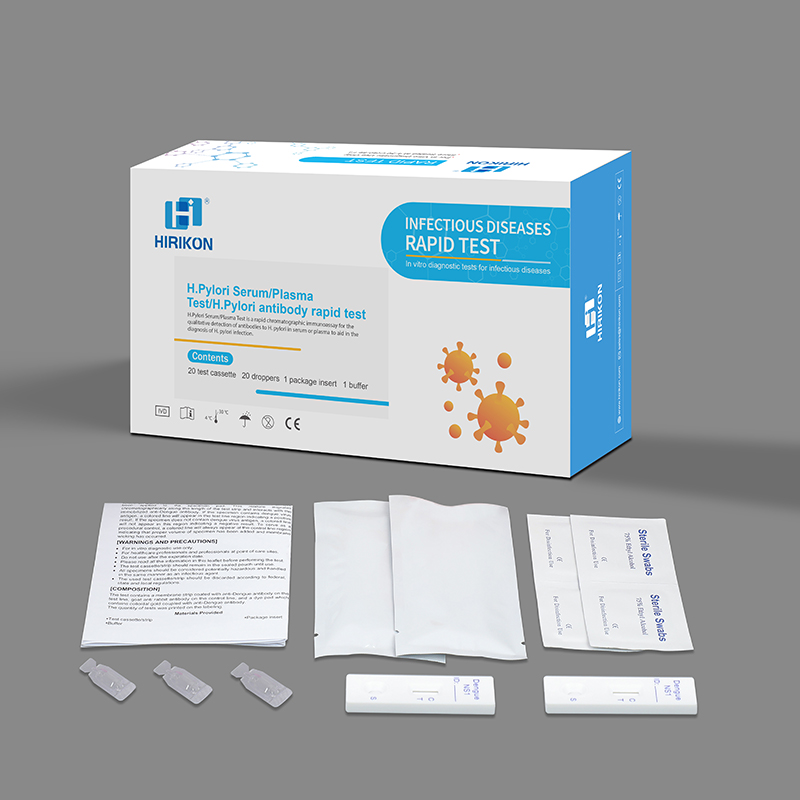
H.Pylori Serum/Plasma Test
Place of Origin:China
Brand Name:HIRIKON
Power Source:Manual
Warranty:2 Year
After-sale Service:Online technical support
Material:Plastic
Shelf Life:2years
Quality Certification:ce
Instrument classification:Class II
Safety standard:None
Product Type:H.Pylori Antigen Test Detector Stool One Step
Specimen:Whole blood/serum/plasma
Size:2.5mm 3mm 4mm
Usage:Professional Testing
Application:vitro diagnostic reagents
Package:Individual Foil Pouch
Name:IVD Reagent
Accuracy:Over 99%
CE approved diagnostic kit for h.pylori antigen rapid test card kit
- Intended Use
The reagent is used to detect the HP antibody in serum / plasma / whole blood qualitatively.
Helicobacter Pylori (HP) grows in gastric mucus deep layer, surface of gastric mucosa, and mostly in gastric antrum, gastric pit, epithelial deep fold and gland cavity. There will be a short-term acute gastritis symptom with epigastric pain, nausea, emesis and flatulence after helicobacter pylori enter the stomach. The most common infection is chronic gastric inflammation with no obvious symptoms, which will cause duodenal ulcer and gastric ulcer. HP is a pathogenic factor of stomach cancer, for causing Induction of bacterial proliferation, Changes of gastric mucosa, decrease of hydrochloric acid in gastric juice. Over 90% of duodenal ulcer is found with HP, over 70% of gastric ulcer is found with HP and over 60% of chronic gastritis is associated with HP. - Test Procedure
Instructions must be read entirely before taking the test. Allow the test device controls to equilibrate to room temperature for 30 minutes (20°C -30°C) prior to testing. Do not open the inner packaging until ready, it must be used in one hour if opened (Humidity: 20%~90%, Temp: 10°C-50°C)
Strip and Cassette:
1. Take off the outer packing, put the strip/cassette onto the desk with the sample adding area of the strip/ sample well of the cassette up.
2. Serum / Plasma: Drop 1 drop (25μl) of serum or plasma vertically into the sample pad of strip/into the sample well of cassette. Add about 2 drops (80μl-100μl) of sample buffer onto the sample pad of strip /into the sample well of cassette.
3. Whole blood: Drop 2 drops (50μl) of whole blood vertically into the sample pad of strip/into the sample well of cassette. Add about 2 drops (80μl-100μl) of sample buffer onto the sample pad of strip into the sample well of cassette. - Result Judgment
POSITIVE: If two color bands are visible and the test band is equal to or darker than the control band,the FSH in the sample is at or above the detection sensitivity of 25 mIU/mL.
NEGATIVE: Only one color band appears on the control region or the test band appears but is lighter than the control band. The FSH concentration of the sample is below the detection sensitivity of 25 mIU/mL
INVALID: No red lines appear or control line fails to appear, indicating that the operator error or reagent failure. Verify the test procedure and repeat the test with a new testing device.







Reviews
There are no reviews yet.